Published TCIMAIL newest issue No.199
Maximum quantity allowed is 999
Griess Reagent Kit for Measuring Nitrite Ions
Nitric oxide (NO) plays diverse roles in processes such as vasodilation, neurotransmission, immune responses, and controlling cell proliferation. It is also associated with numerous diseases, including cardiovascular disease, neurodegenerative disorders, and cancer.1)
Griess Reagent Kit (1) is a colorimetric reagent kit for the quantification of nitrite ions (NO2⁻), a stable metabolite of NO. NO2⁻ in the sample reacts with sulfanilamide of 1 to form a diazonium salt. This salt then reacts with N-(1-naphthyl)ethylenediamine (NED) in 1 to produce the reddish-purple diazo dye, which has a maximum absorption peak near 540 nm. By measuring this color intensity, the NO2⁻ concentration can be quantified, and consequently, the NO concentration can be determined indirectly.2,3)
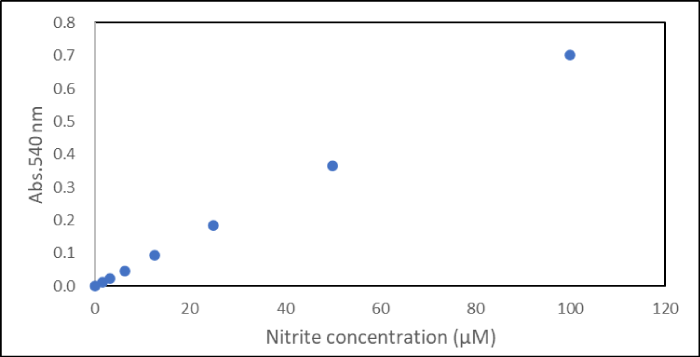
Figure. The measurement result of NO2⁻ using 1 taken in-house.
Related Products
- G0667
- Griess Reagent Kit
- S0833
- Sulfanilamide
- U0107
- Sulfanilic Acid
- N0869
- N-(1-Naphthyl)ethylenediamine Dihydrochloride
Related Product Category Page
References
- 1) Nitric oxide signaling in health and disease
- 2) Evaluation of novel Griess-reagent candidates for nitrite sensing in aqueous media identified via molecular fingerprint searching
- 3) Automated determination of nitrate plus nitrite in aqueous samples with flow injection analysis using vanadium (III) chloride as reductant

