Maximum quantity allowed is 999
Please select the quantity
CAS RN: 1109-15-5 | 제품번호: T2313
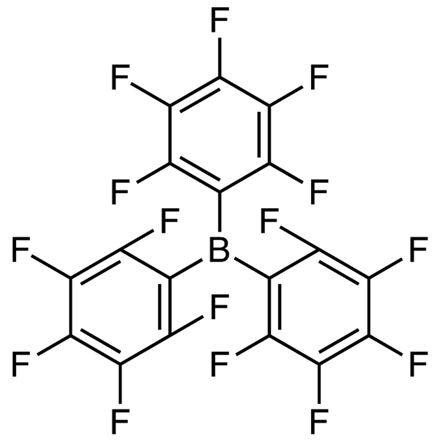
Tris(pentafluorophenyl)borane
순도/분석 방법: >98.0%(NMR)
동의어의:
제품문서:
•본 제품의 재고는 일본 본사의 재고입니다. 주문시, 5일안에 실험실까지 도착됩니다.
•본건의 원가격은 한국 대리점의 예상 판매가격입니다.자세한 정보가 필요하시면 연락해 주십시오.( SEJIN CI Co., Ltd. (한국총대리점) 전화 : 02-2655-2480 이메일 : sales@sejinci.co.kr)
•보관 조건은 예고없이 변경 될 수 있습니다. 제품 보관 조건의 최신 자료는 홈페이지에 기재되어 있으니 양해 부탁드립니다.
•본건의 원가격은 한국 대리점의 예상 판매가격입니다.자세한 정보가 필요하시면 연락해 주십시오.( SEJIN CI Co., Ltd. (한국총대리점) 전화 : 02-2655-2480 이메일 : sales@sejinci.co.kr)
•보관 조건은 예고없이 변경 될 수 있습니다. 제품 보관 조건의 최신 자료는 홈페이지에 기재되어 있으니 양해 부탁드립니다.
| 제품번호 | T2313 |
Purity/Analysis Method 
|
>98.0%(NMR) |
| M.F. / M.W. | C__1__8BF__1__5 = 511.98 |
| 물리적 상태 (20 ℃) | Solid |
보관 조건 
|
Frozen (<0°C) |
| 불활성 가스 하에서 보관 | Store under inert gas |
| 피해야 할 조건 | Hygroscopic,Heat Sensitive |
| CAS RN | 1109-15-5 |
| Reaxys-RN | 2931347 |
| PubChem Substance ID | 87558752 |
| Merck Index (14) | 9755 |
| MDL 번호 | MFCD00269813 |
규격표
| Appearance | White to Gray to Brown powder to crystal |
| Purity(NMR) | min. 98.0 atom% |
| Water | max. 1.0 % |
물성치(참고치)
| mp | 128 °C |
| Maximum Wavelength | 306 nm (Toluene) |
| 용해성 (용해) | Toluene |
GHS
| 픽토그램 |

|
| 신호 워드 | Warning |
| 위험물 및 유해 등록 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 주의 사항 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
법규 정보
운송 정보
| HS 번호* | 2931.90-000 |
Application
Deuteration of Aromatic and Heteroamomatic Compounds Catalyzed by Tris(pentafluorophenyl)borane

Experimental procedure: N,N-Dibenzylaniline (140 mg, 0.513 mmol), tris(pentafluorophenyl)borane (17.0 mg, 0.033 mmol), D2O (306 mg, 15.3 mmol) are placed in a sealed tube. The reaction mixture is stirred at 80 °C for 24 h. After the reaction is completed, the reaction mixture is purified by silica gel column chromatography (cyclohexane:ethylacetate = 20:1) to afford N,N-dibenzylaniline-2,4,6-d3 (134 mg) in 95% yield.
References
- B(C6F5)3‑Catalyzed Regioselective Deuteration of Electron-Rich Aromatic and Heteroaromatic Compounds
Application
High Throughput Sequence-controlled Oligosiloxane Synthesis

References
- By-Product-Free Siloxane-Bond Formation and Programmed One-Pot Oligosiloxane Synthesis
Application
Frustrated Lewis Pair (FLP)-induced Hydrogenations of Silyl Enol Ethers

References
- Heterolytic dihydrogen activation with the 1,8-bis(diphenylphosphino)naphthalene/B(C6F5)3 pair and its application for metal-free catalytic hydrogenation of silyl enol ether
Application
Tri(cyclohexa-2,5-dien-1-yl)silane: A Stable and Easy-to-handle Surrogate of Monosilane (SiH4)

Synthesis of multisubstituted silanes:
In a glove box, a Schlenk tube is charged with an alkene (0.300 mmol). In two vials are weighed B(C6F5)3 (7.7 mg, 15 µmol) and tri(cyclohexa-2,5-dien-1-yl)silane (1, 28.0 mg, 0.105 mmol). All the sealed containers are taken out of the glove box, and the Schlenk tube is connected to a N2 line. Under a N2 counterflow are subsequently added 0.3 mL of CH2Cl2, 1 then B(C6F5)3. The tube is completely sealed and stirred at room temperature for 24 h. After this time, two drops of triethylamine are added to the solution under an N2 counterflow, and the resulting mixture is stirred for 5 min before being filtered over a short alumina column (elution with CH2Cl2). After evaporation of all volatiles, the crude product is purified by flash column chromatography to give the multisubstituted silane 3.
In a glove box, a Schlenk tube is charged with an alkene (0.300 mmol). In two vials are weighed B(C6F5)3 (7.7 mg, 15 µmol) and tri(cyclohexa-2,5-dien-1-yl)silane (1, 28.0 mg, 0.105 mmol). All the sealed containers are taken out of the glove box, and the Schlenk tube is connected to a N2 line. Under a N2 counterflow are subsequently added 0.3 mL of CH2Cl2, 1 then B(C6F5)3. The tube is completely sealed and stirred at room temperature for 24 h. After this time, two drops of triethylamine are added to the solution under an N2 counterflow, and the resulting mixture is stirred for 5 min before being filtered over a short alumina column (elution with CH2Cl2). After evaporation of all volatiles, the crude product is purified by flash column chromatography to give the multisubstituted silane 3.
References
- Formal SiH4 chemistry using stable and easy-to-handle surrogates
Application
Metal-Free Hydrogenation of Ketone by Frustrated Lewis Pairs (FLPs)

Typical procedure (R, R’ = n-Pr): 4-Heptanone (1.00 g, 8.76 mmol) is weighed into a 125 mL reactor. Subsequently, B(C6F5)3 (0.224 g, 0.43 mmol) dissolved in Et2O (14.3 mg, 20 mL, 0.19 mol) is added to the reactor. The reactor is sealed and attached to a hydrogen gas line. The flask is purged ten times at 15 atm with hydrogen gas. The reactor is then pressurized with 60 atm hydrogen gas and placed in an oil bath for 12 h at 70 °C. The reactor is slowly vented and all the volatiles are collected by vacuum distillation while cooling the collected distillate with liquid nitrogen. The solvent is removed by applying a gentle stream of N2 gas to give 4-heptanol (886 mg, 87% yield, and 99 % conversion determined by 1H NMR).
References
- T. Mahdi, D. W. Stephan, J. Am. Chem. Soc. 2014, 136, 15809.

- Highlighted in Chem. Eng. News 2014, 92 (44), 8.

Application
Metal-Free Hydrogenation of N-Phenyl Aromatic Rings

Typical procedure (Entry 1): A 25 mL glass bomb equipped with Teflon screw cap is charged with a solution of B(C6F5)3 (37.9 mg, 1 eq.) and N-isopropylaniline (10.0 mg, 0.074 mmol) in toluene (1 mL). The reaction tube is degassed three times through a freeze-pump-thaw cycle on the vacuum/H2 line and filled with H2 (4 atm) at -196 °C. The reaction bomb is placed in a 110 °C oil bath for 36 h. The toluene is removed under reduced vacuum to yield a white precipitate. The product is washed with pentane (2 x 2 mL) and dried under reduced pressure to give [iPrNH2Cy][HB(C6F5)3] (93 %).
References
- 1)T. Mahdi, Z. M. Heiden, S. Grimme, D. W. Stephan, J. Am. Chem. Soc. 2012, 134, 4088.

- 2)D. W. Stephan, G. Erker, Angew. Chem., Int. Ed. 2010, 49, 46.

Application
Metal-free Hydrogenation of Imines Catalyzed by B(C6F5)3

Typical Procedure: In a glovebox, B(C6F5)3 (18.2 mg, 0.0355 mmol, 20 mol%) is dissolved in dry diisopropylamine (2.5 mL, 1.8 g, 17 mmol) and the solution is added to N-benzylidene-tert-butylamine (28.7 mg, 0.177 mmol, 1 eq.). The resulting solution is transferred to a 25 mL bomb with a sealable Teflon tape and magnetic stirbar. The reaction vessel is sealed, removed from the glovebox and stirred at 100°C for 24 h after which it is cooled to room temperature. The reaction mixture is quenched by the addition of silica followed by elution through a short silica column. The filtrate is concentrated in vacuo to give the desired product.
References
PubMed Literature
제품기사
[Product Highlights] Deuteration of Aromatic and Heteroamomatic Compounds Catalyzed by Tris(pentafluorophenyl)borane[Product Highlights] A Bisphosphine Usable for Metal-free Hydrogenations
[Research Articles] High Throughput Sequence-controlled Oligosiloxane Synthesis
[Research Articles] Tri(cyclohexa-2,5-dien-1-yl)silane: A Stable and Easy-to-handle Surrogate of Monosilane (SiH4)
[Research Articles] Metal-Free Hydrogenation of Ketone by Frustrated Lewis Pairs (FLPs)
[Research Articles] Metal-Free Hydrogenation of N-Phenyl Aromatic Rings
[Research Articles] Metal-Free Hydrogenation of Imines Catalyzed by B(C6F5)3
제품문서 (분석 차트를 제공하지 못할 수도 있으니 양해 바랍니다.)
SDS
언어를 선택하십시오.
요청한 SDS를 사용할 수 없습니다.
번거롭게 해드려 죄송하지만 이 양식 보다 문의 해주십시오.
규격표
시험성적서, 각종 증명서
롯트 번호를 입력하십시오.
잘못된 로트 번호가 입력되었습니다.
샘플 시험성적서
시험성적서 샘플을 다운로드할 수 있습니다. 샘플은 반드시 최신 로트의 결과를 나타내는 것은 아닙니다.
본 제품의 샘플시험성적서는 현재 준비되어 있지 않습니다.
분석 차트

롯트 번호를 입력하십시오.
잘못된 로트 번호가 입력되었습니다.
죄송합니다만 찾으시는 분석차트는 없습니다.




