Maintenance Notice (12:30 AM - 4:00 AM June 8, 2025): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Published TCIMAIL newest issue No.198
Maximum quantity allowed is 999
| Size | Unit Price | Belgium | Japan* | Quantity |
|---|---|---|---|---|
| 1G |
€100.00
|
4 | 31 |
|
| 5G |
€333.00
|
2 | 4 |
|
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
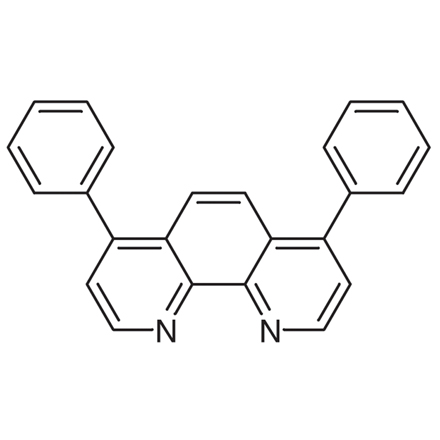
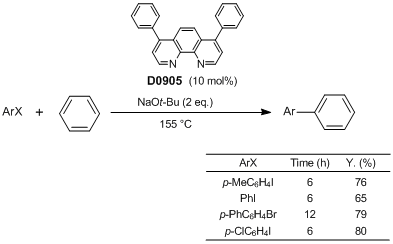
| Product Number | D0905 |
| Purity / Analysis Method | >99.0%(T) |
| Molecular Formula / Molecular Weight | C__2__4H__1__6N__2 = 332.41 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Packaging and Container | 1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 1662-01-7 |
| Reaxys Registry Number | 261048 |
| PubChem Substance ID | 87567505 |
| SDBS (AIST Spectral DB) | 3200 |
| MDL Number | MFCD00004976 |
| Appearance | White to Orange to Green powder to crystal |
| Purity(Nonaqueous Titration) | min. 99.0 % |
| Purity(HPLC) | min. 98.0 area% |
| Sensitiveness | Abs min.0.36(near 533nm) in the presence of Fe(1 ppm) |
| Melting Point | 221 °C |
| Solubility in water | Slightly soluble |
| Solubility (soluble in) | Ethanol, Acetone, Benzene |
| EC Number | 216-767-1 |
| RTECS# | SF8427000 |
| HS Number | 2933998090 |

The requested SDS is not available.
Please Contact Us for more information.
A sample C of A for this product is not available at this time.
The requested analytical chart is not available. Sorry for the inconvenience.