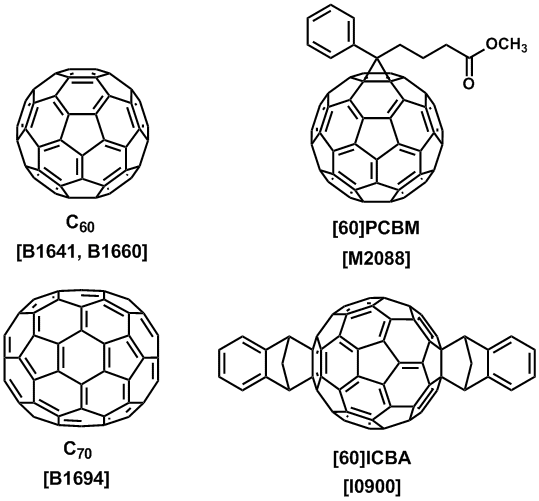
Addition reactions and other chemical modifications of fullerenes easily produce fullerene derivatives. Precise structure analyses of these derivatives are possible because they are molecular species. Non-derivatized fullerenes are poorly soluble in similarity to the other nanocarbon materials. However, we can introduce soluble functional groups to form solution-processible electronic materials. Phenyl-C61-butyric acid methyl ester ([60]PCBM) and indene-C60 bisadduct (ICBA) are useful organic semiconductors for fabricating a solution-processible electronic device.5,6) These fullerene derivatives are n-type organic semiconductors for organic photovoltaics (OPV) by mixing with a p-type conjugated polymer.7) An application of a fullerene derivative for organic transistors was also reported.8) A complexation of C60 with tetrakis(dimethylamino)ethylene (TDAE) gives a charge transfer complex (TDAE-C60), which is an organic magnet at low temperature.9)
Although a chemical modification of the outer surface of fullerene provides PCBM or ICBA, we can introduce a small component to the inner side of fullerene. For instance, fullerenes can encapsulate a metal atom on the inner side, when the fullerenes are produced in the presence of the metal. This is the so-called metal-encapsulated fullerene described as MC60. The encapsulation modifies the electronic state10) and chemical reactivity of fullerene.11) On the other hand, water-encapsulated fullerene (H2OC60) was also reported. A publication described that an organic synthetic procedure gave open-caged C60 which could then encapsulate one water molecule. A following chemical modification could close the cage to from water-encapsulated fullerene H2OC60.12)
Sign up for free shipping on all website orders, no minimum required, and get exclusive coupons!
Users can now ask questions to the AI chatbot by clicking the chatbot icon in the bottom-right corner of the website.



![Fullerene C60 (purified by sublimation) [for organic electronics] Fullerene C60 (purified by sublimation) [for organic electronics]](/medias/F1232.jpg?context=bWFzdGVyfHJvb3R8Mzg4ODd8aW1hZ2UvanBlZ3xhR1V6TDJobE5TODVNVGMyTkRjNE1qZzFPRFUwTDBZeE1qTXlMbXB3Wnd8NWZhZWYzYTVhNWRiY2U5Y2NlNzk5ZGU3MzRhN2QzNDEwMDk4OWJhOWNkZDMzZjczYWRhNWE3MzQ3ZTlhZTBkNQ)
![[6,6]-Phenyl-C61-butyric Acid Methyl Ester [for organic electronics] [6,6]-Phenyl-C61-butyric Acid Methyl Ester [for organic electronics]](/medias/P2682.jpg?context=bWFzdGVyfHJvb3R8NDk5MjV8aW1hZ2UvanBlZ3xhRFU1TDJnME5TODRPVE15TVRRME56QTVOall5TDFBeU5qZ3lMbXB3Wnd8NTU1ZTExOTA3MjFjYmRhYjIyMzAyY2Y0ZGM0ZjYzNjlhOTc4ZGY2ZDJiYjU0ZjUxZmIxNTQxZGI1MWY2NDNjMg)
![[6,6]-Phenyl-C61-butyric Acid Methyl Ester [6,6]-Phenyl-C61-butyric Acid Methyl Ester](/medias/M2088.jpg?context=bWFzdGVyfHJvb3R8NTAwMDZ8aW1hZ2UvanBlZ3xhR1JtTDJnNU15ODRPVE14TkRrNU56WTVPRGcyTDAweU1EZzRMbXB3Wnd8MGUwZjIyZTEzMWM5MWYwMTU5ZjRlZWJmYzY4ZGQ4NGRjZTYxNWZlYTNkNGU2ZjE3NWRhNTUwM2NjZDVhNzcxOA)
![Fullerene C70 (purified by sublimation) [for organic electronics] Fullerene C70 (purified by sublimation) [for organic electronics]](/medias/F1233.jpg?context=bWFzdGVyfHJvb3R8NTc2MTN8aW1hZ2UvanBlZ3xhREEwTDJoa015ODRPVE13TnpJME1qZ3lNems0TDBZeE1qTXpMbXB3Wnd8MjRiMGMxYWUxNzAyZmYyNTFkODViMTFkMjUxOWViMjAyN2QwMGU1ZmRhMjkzMTBiODlhMjViNjJhMjIzZTUwZQ)
![[6,6]-Phenyl-C71-butyric Acid Methyl Ester (mixture of isomers) [for organic electronics] [6,6]-Phenyl-C71-butyric Acid Methyl Ester (mixture of isomers) [for organic electronics]](/medias/P2683.jpg?context=bWFzdGVyfHJvb3R8NTA4Njd8aW1hZ2UvanBlZ3xhRGs0TDJnME5DODRPVE15TVRRME56YzFNVGs0TDFBeU5qZ3pMbXB3Wnd8ODY2ZWFlY2Y5NjUxN2E5ODA5NWRkZTkwMzViMWJkYjM5ZmMwYmI0MzAxMWJiNzRmNGUxMDE3Zjc2MTFhN2E4Yw)


![N,2-Diphenyl[60]fulleropyrrolidine (contains 5% Hexane at maximum) N,2-Diphenyl[60]fulleropyrrolidine (contains 5% Hexane at maximum)](/medias/D5757.jpg?context=bWFzdGVyfHJvb3R8NDU5MzZ8aW1hZ2UvanBlZ3xhR0ppTDJnNFppODRPVE13TkRVd09EWTJNakEyTDBRMU56VTNMbXB3Wnd8ZTk1ZTBmODllOTNjYTA0MDcyNjgwZjc3NWFhYzg0MjAxZTc2MGI2YmJjNDlhMTU2ODQ5NDhjZWFjMzM1ZGFiOQ)
![[6,6]-Phenyl-C71-butyric Acid Methyl Ester (mixture of isomers) [6,6]-Phenyl-C71-butyric Acid Methyl Ester (mixture of isomers)](/medias/M2550.jpg?context=bWFzdGVyfHJvb3R8NTAwNTV8aW1hZ2UvanBlZ3xhRGRtTDJnek5DODRPVE14TlRnNU9UUTNOREl5TDAweU5UVXdMbXB3Wnd8YzU4ZDkwMDkzMDQ3NTI4MjI2NTE4YmVlNWZiNDc2YTNjYjVlMTNjNTY3ZDJmNGM0ZTgxMDM3M2Y5MGJlZDVjNQ)

![[6,6]-Phenyl-C61-butyric Acid Butyl Ester [6,6]-Phenyl-C61-butyric Acid Butyl Ester](/medias/P2013.jpg?context=bWFzdGVyfHJvb3R8NDgzNzB8aW1hZ2UvanBlZ3xhREF5TDJoaU1TODRPVE15TVRFME9ESTFNalEyTDFBeU1ERXpMbXB3Wnd8Y2IyYjc5NWZhZTU5Y2U0OTQxNjkyZDQwNjNhZDRhMTc3NDFkNmE2ZDZhNTcxZmYwMGIyMjJjYWMxYWRiOGU0Yg)