Sign up for free shipping on all website orders, no minimum required, and get exclusive coupons!
Users can now ask questions to the AI chatbot by clicking the chatbot icon in the bottom-right corner of the website.
Maximum quantity allowed is 999
* Items in stock locally typically ship the same day of ordering. Items from Japan stock are able to ship from a US warehouse within 2 weeks after ordering. For additional estimated shipment times, please refer to the Shipping Simulation tool. Note: Excludes regulated items and items that ship on ice.
* To send your quote request for bulk quantities, please click on the "Request Quote" button. Please note that we cannot offer bulk quantities for some products.
*TCI frequently reviews storage conditions to optimize them. Please note that the latest information on the storage temperature for the products is described on our website.
| Product Number | D3546 |
Purity / Analysis Method 
|
>97.0%(HPLC) |
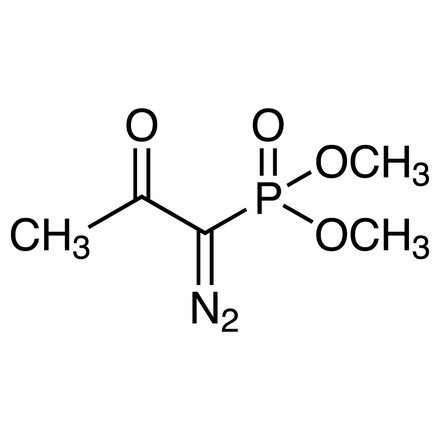
| Molecular Formula / Molecular Weight | C__5H__9N__2O__4P = 192.11 |
| Physical State (20 deg.C) | Liquid |
Storage Temperature 
|
Refrigerated (0-10°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive,Heat Sensitive |
| CAS RN | 90965-06-3 |
| Reaxys Registry Number | 4247670 |
| PubChem Substance ID | 87559944 |
| Merck Index (14) | 1177 |
| MDL Number | MFCD07368360 |
| Appearance | Light yellow to Brown clear liquid |
| Purity(HPLC) | min. 97.0 area% |
| Specific Gravity (20/20) | 1.28 |
| Refractive Index | 1.48 |
| HS Number | 2931.90.9052 |

To a methanol (10 mL) solution of undecanal (0.20 g, 1.2 mmol) was added potassium carbonate (0.32 g,2.4 mmol) and Ohira-Bestmann reagent (0.27 g, 1.4 mmol) at room temperature. The reaction mixture was stirred overnight at room temperature, then diluted with diethyl ether and washed with saturated aqueous sodium bicarbonate, and dried by sodium sulfate. The organic layer was concentrated under reduced pressure. The resulting crude product was purified by column chromatography (hexane:toluene = 3:1 on silica gel) to give 1 as a colorless liquid (0.11 g, 56% yield).
The reaction mixtures were monitored by 1H NMR (CDCl3).
1H NMR (400 MHz, CDCl3); δ 2.18 (t, 2H, J = 7.1 Hz), 1.94 (s, 1H), 1.57-1.46 (m, 2H), 1.44-1.33 (m, 2H), 1.33-1.19 (m, 12H), 0.88 (t, 3H, J = 6.4 Hz).
13C NMR (101 MHz, CDCl3); δ 85.0, 68.2, 32.0, 29.7, 29.7, 29.5, 29.3, 28.9, 28.6, 22.8, 18.5, 14.3.


The requested SDS is not available.
Please Contact Us for more information.
A sample C of A for this product is not available at this time.

The requested analytical chart is not available. Sorry for the inconvenience.