Sign up for free shipping on all website orders, no minimum required, and get exclusive coupons!
Users can now ask questions to the AI chatbot by clicking the chatbot icon in the bottom-right corner of the website.
Maximum quantity allowed is 999
* Items in stock locally typically ship the same day of ordering. Items from Japan stock are able to ship from a US warehouse within 2 weeks after ordering. For additional estimated shipment times, please refer to the Shipping Simulation tool. Note: Excludes regulated items and items that ship on ice.
* To send your quote request for bulk quantities, please click on the "Request Quote" button. Please note that we cannot offer bulk quantities for some products.
*TCI frequently reviews storage conditions to optimize them. Please note that the latest information on the storage temperature for the products is described on our website.
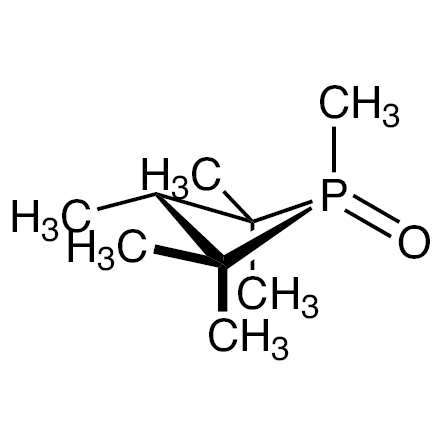
| Product Number | H1766 |
Purity / Analysis Method 
|
>98.0%(GC) |
| Molecular Formula / Molecular Weight | C__9H__1__9OP = 174.22 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Moisture Sensitive |
Packaging and Container 
|
1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 33530-51-7 |
| Reaxys Registry Number | 1072177 |
| PubChem Substance ID | 468592039 |
| MDL Number | MFCD32857307 |
| Appearance | White to Light yellow powder to crystal |
| Purity(GC) | min. 98.0 % |
| Melting point | 171.0 to 175.0 °C |
| NMR | confirm to structure |
| Melting Point | 174 °C |
| HS Number | 2931.49.0080 |

To a 3-necked 50 mL flask was charged with 1-bromo-2-nitrobenzene (2.006 g, 9.931 mmol, 1.0 equiv.), phenylboronic acid (1.332 g, 10.92 mmol, 1.1 equiv.) and anti-1,2,2,3,4,4-hexamethylphosphetane 1-oxide (0.259 g, 1.49 mmol, 0.15 equiv.). The flask was evacuated on a Schlenk line, and backfilled with argon (3 times). The flask was then charged dry CPME (20 mL), followed by phenylsilane (2.4 mL, 20 mmol, 2.0 equiv.). The mixture was then heated to reflux and stirred for 4 h. The reaction mixture was cooled to room temperature and washed with 1 mol/L NaOH aqueous solution (40 mL). The organic layer was separated and the aqueous layer was extracted with ethyl acetate (20 mL). The combined organic layers were washed with brine (20 mL), dried over Na2SO4 and filtered. The solvent was removed under reduced pressure. The residue was purified by column chromatography (eluent: dichloromethane/ethyl acetate/hexane, 20/1/79) to obtain 1 as a white solid (2.20 g, 89.3%).
The reaction mixture was monitored by GC.
CPME was dried over MS4A before use.
1H NMR (400 MHz, CDCl3); δ 7.54 (dd, J = 7.8, 1.4 Hz, 1H), 7.32–7.36 (m, 2H), 7.26–7.28 (m, 1H), 7.16–7.18 (m, 3H), 7.06 (t, J = 7.3 Hz), 6.75 (ddd, J = 7.8, 7.3, 1.4 Hz), 5.79 (brs, 1H).
13C NMR (101 MHz, CDCl3); δ 141.7, 141.5, 133.1, 129.6, 128.2, 122.8, 121.0, 120.4, 115.9, 112.3.
The requested SDS is not available.
Please Contact Us for more information.
A sample C of A for this product is not available at this time.

The requested analytical chart is not available. Sorry for the inconvenience.