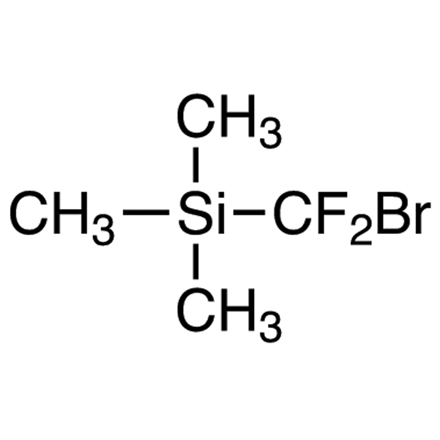
An Effective Precursor of a Difluorocarbene to Introduce Difluoromethylene Groups
Difluoromethylenation of alkynes: under a N
2 atmosphere, alkynes (0.5 mmol, 1.0 eq.), (bromodifluoromethyl)trimethylsilane (152 mg, 0.75 mmol, 1.5 eq.),
tetrabutylammonium bromide (4.8 mg, 0.015 mmol, 0.03 eq.), and toluene (2.0 mL) are added into an oven-dried pressure tube at room temperature. After being heated at 110 °C for 2 h, the reaction mixture is cooled to rt and poured into a saturated Na
2CO
3 solution (5 mL), followed by extraction with Et
2O (2 × 15 mL). The organic layers are combined and dried over anhydrous K
2CO
3. After the removal of the solvents
in vacuo, the residue is subjected to column chromatography (silica gel; petroleum ether : Et
3N = 40 : 1) to afford the desired products. [Note: these products are sensitive to acid, so the column must be eluted previously with petroleum ether : Et
3N = 10 : 1)].