盲盒好礼,精彩继续 | 轻松扫码查看产品文档 | TCIMAIL No.198 已上新 | TCI试剂——品质可靠,值得信赖
订购方法?联系方式:021-67121386 / Sales-CN@TCIchemicals.com
Maximum quantity allowed is 999
请选择数量
CAS RN: 81290-20-2 | 产品编码: T1570
(Trifluoromethyl)trimethylsilane [Trifluoromethylating Reagent]
![(Trifluoromethyl)trimethylsilane [Trifluoromethylating Reagent] No-Image](/medias/T1570.jpg?context=bWFzdGVyfHJvb3R8NzU0MTB8aW1hZ2UvanBlZ3xhRGRsTDJoaVpTODRPVEl6TkRFM05qY3pOelU0TDFReE5UY3dMbXB3Wnd8YjdiMmNiNWZiODExMzE5ZDE3Mzg5MDVmNDYwOGIzYTM1OGQxYzY1YWZhZTlkNTg3OTczMmI0MmY2YzhhNTQzZg)
* 点击“查询”可查看预计发货日期,仅供参考。
* 无具体发货日期的情况,如:显示“8个工作日后发货”,将在您订购日起的8个工作日后发货。
* 我们将以最优方式从上海/天津两大仓库发货。国内库存不足,需两周左右向日本总部调货。
* 对于可分装产品,11:30前的订单,当天发货;11:30后的订单,隔天发货。
* 如需大包装,请点击“大包装询价”按钮(对于某些产品我们无法提供大包装)。
* TCI会经常复审储藏条件以对其进行优化,请以在线目录为准,敬请留意。
* 更多信息,请联系营业部:021-67121386 / Sales-CN@TCIchemicals.com 。任何货期、规格或包装方面的需求,请联系我们 。
* 无具体发货日期的情况,如:显示“8个工作日后发货”,将在您订购日起的8个工作日后发货。
* 我们将以最优方式从上海/天津两大仓库发货。国内库存不足,需两周左右向日本总部调货。
* 对于可分装产品,11:30前的订单,当天发货;11:30后的订单,隔天发货。
* 如需大包装,请点击“大包装询价”按钮(对于某些产品我们无法提供大包装)。
* TCI会经常复审储藏条件以对其进行优化,请以在线目录为准,敬请留意。
* 更多信息,请联系营业部:021-67121386 / Sales-CN@TCIchemicals.com 。任何货期、规格或包装方面的需求,请联系我们 。
技术规格
| Appearance | Colorless to Light yellow clear liquid |
| Purity(GC) | min. 97.0 % |
物性(参考值)
| 沸点 | 55 °C |
| 闪点 | -32 °C |
| 比重 | 0.96 |
| 折射率 | 1.33 |
GHS
| 象形图 |

|
| 信号词 | 危险 |
| 危险性说明 | H225 : 高度易燃液体和蒸气。 |
| 防范说明 | P501 : 将内装物/容器送到批准的废物处理厂处理。 P240 : 容器和装载设备接地/等势联接。 P210 : 远离热源/火花/明火/热表面。禁止吸烟。 P233 : 保持容器密闭。 P243 : 采取防止静电放电的措施。 P241 : 使用防爆的电气/通风/照明设备。 P242 : 只能使用不产生火花的工具。 P280 : 戴防护手套/戴防护眼罩/戴防护面具。 P370 + P378 : 火灾时:使用干砂、干粉或抗醇泡沫灭火。 P303 + P361 + P353 : 如皮肤(或头发)沾染:立即脱掉所有沾污的衣物。用水清洗皮肤/淋浴。 P403 + P235 : 存放在通风良好的地方。保持低温。 |
相关法规
| 新化学物质备案回执号 | B1A232215762 |
运输信息
| UN编号 | UN1993 |
| 类别 | 3 |
| 包装类别 | II |
| 监管条件代码(*) |
应用
Synthesis of Trifluoromethylketones from Weinreb Amides
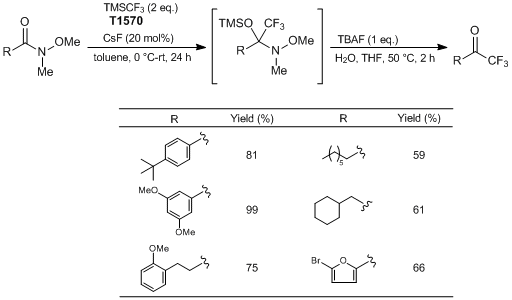
Typical Procedure:
To a 50 mL round bottom flask equipped with a stir bar is added CsF (0.1512 g, 1.0 mmol, 0.2 eq.). Toluene (2.5 mL) is added to the flask, followed by 4-(tert-butyl)-N-methoxy-N-methylbenzamide (1.11 g, 5.0 mmol, 1 eq.). The flask is sealed with a septum equipped with an inlet needle as an exit valve. The flask is cooled to 0 °C for 10 minutes. Once cooled, TMSCF3 (1.42 g, 10.0 mmol, 2 eq.) is added to the reaction mixture dropwise over a period of ≈ 10 minutes. After completion of addition, the reaction mixture is allowed to stir at 0 °C for 10 minutes. The cooling bath is removed and the reaction mixture is allowed to stir at room temperature overnight. (Upon reaching room temperature, the reaction occurs and is mildly exothermic and gas is evolved.) Reaction progress is monitored by 1H NMR.
Once complete conversion to the silylated intermediate is confirmed, water (5 mL) followed by TBAF (5 mL, 1 M in THF, 1 eq.) is added to the reaction flask. The flask is equipped with a reflux condenser, open to air. The contents are then heated to 50 °C, and allowed to stir at that temperature for 2 hours. Once cooled to room temperature, the reaction mixture is diluted with Et2O (≈ 30 mL), and transferred to a separatory funnel. The organic layer is washed with deionized water (3 × 30 mL), followed with a brine solution (1 × 30 mL). The organic layer is dried with Na2SO4 and the solvent is removed in vacuo by rotary evaporation to yield crude trifluoromethylketone. Further purification is accomplished by flash chromatography (hexane : EtOAc = 8 : 2) to produce the pure CF3 ketone as an orange solid (0.935 g, Y. 81%).
To a 50 mL round bottom flask equipped with a stir bar is added CsF (0.1512 g, 1.0 mmol, 0.2 eq.). Toluene (2.5 mL) is added to the flask, followed by 4-(tert-butyl)-N-methoxy-N-methylbenzamide (1.11 g, 5.0 mmol, 1 eq.). The flask is sealed with a septum equipped with an inlet needle as an exit valve. The flask is cooled to 0 °C for 10 minutes. Once cooled, TMSCF3 (1.42 g, 10.0 mmol, 2 eq.) is added to the reaction mixture dropwise over a period of ≈ 10 minutes. After completion of addition, the reaction mixture is allowed to stir at 0 °C for 10 minutes. The cooling bath is removed and the reaction mixture is allowed to stir at room temperature overnight. (Upon reaching room temperature, the reaction occurs and is mildly exothermic and gas is evolved.) Reaction progress is monitored by 1H NMR.
Once complete conversion to the silylated intermediate is confirmed, water (5 mL) followed by TBAF (5 mL, 1 M in THF, 1 eq.) is added to the reaction flask. The flask is equipped with a reflux condenser, open to air. The contents are then heated to 50 °C, and allowed to stir at that temperature for 2 hours. Once cooled to room temperature, the reaction mixture is diluted with Et2O (≈ 30 mL), and transferred to a separatory funnel. The organic layer is washed with deionized water (3 × 30 mL), followed with a brine solution (1 × 30 mL). The organic layer is dried with Na2SO4 and the solvent is removed in vacuo by rotary evaporation to yield crude trifluoromethylketone. Further purification is accomplished by flash chromatography (hexane : EtOAc = 8 : 2) to produce the pure CF3 ketone as an orange solid (0.935 g, Y. 81%).
References
应用
Nucleophilic Trifluoromethylation

Reference
应用
Difluorocarbene Precursor

Reference
- Synthesis of gem-difluorinated cyclopropanes and cyclopropenes: trifluoromethyltrimethylsilane as a difluorocarbene source
参考文献
产品文档 (部分产品的分析图谱无法提供,敬请谅解。)
化学品安全说明书(SDS)
请选择语言。
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
示例 CoA
可下载CoA示例。注:该示例不一定是最新批次的CoA。
目前没有该产品的 CoA 示例。
分析图谱
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
很抱歉,您搜索的分析图谱无法提供。



![(Trifluoromethyl)trimethylsilane [Trifluoromethylating Reagent] (Trifluoromethyl)trimethylsilane [Trifluoromethylating Reagent]](/_ui/responsive/theme-tci/images/missing_product_zoom.jpg)


