Caution Notice:
It has come to our notice that certain fraudulent individuals or entities are misusing our Company’s name and TCI’s registered trademarks by promoting and offering for sale regulated and hazardous chemical substances, including Potassium Cyanide, through online platforms like YouTube. We hereby categorically clarify that TCI has no association or connection whatsoever with the products being displayed or sold in these or similar videos. These products have been falsely represented as being associated with TCI, and the unauthorized use of our trademark and brand name is both illegal and misleading. TCI Chemicals is a responsible global organization committed to adhering to all applicable international and domestic regulatory frameworks. We do not manufacture, distribute, or engage in the sale of regulated chemical substances in India or in any other jurisdiction unless specifically authorized by the relevant laws and regulatory agencies. All our operations and offerings are governed by stringent safety, quality, and compliance protocols in accordance with applicable laws. Further, TCI Chemicals markets and sells its products exclusively through its official website and authorized distributors. We do not sell, endorse, or supply our products through any third-party platforms, unauthorized agents, or resellers. Any individual or organization purchasing products outside these official channels does so at their own risk, and TCI disclaims all responsibility and liability for any consequences arising therefrom. Members of the public, customers, and partners are strongly advised to exercise caution and conduct due diligence before engaging in any transactions involving products claiming association with TCI Chemicals. The official list of our authorized distributors is publicly available and may be accessed at: https://www.tcichemicals.com/IN/en/distributor/index. We are currently pursuing appropriate legal remedies against those misusing our brand, and we reserve all rights available to us under applicable intellectual property and criminal laws. If you become aware of any such fraudulent activity or require clarification, you may reach out to us at: Sales-IN@TCIchemicals.com. Your vigilance and cooperation are essential in safeguarding the public interest and protecting the integrity of the TCI brand.
TCI uses cookies to personalize and improve your user experience. By continuing on our website, you accept the use of cookies. You can change or update your cookiesettings at any time.
Maximum quantity allowed is 999
Cross-coupling Reaction using Transition Metal Catalysts [C-C Bond Formation]
Cross-coupling reactions using late transition metal catalysts represented by nickel and palladium metals have been widely used for introducing various functional groups into unsaturated substances such as aromatic rings, alkenes, alkynes and so on. In these reactions, carbon-carbon bond forming reactions can be performed by the combination of electrophilic carbon species of aryl/vinyl halides and organometallic agents of Grignard reagents and organoboron compounds. Also, the use of nucleophilic hetero atoms such as phenols and amines is efficient to form carbon-hetero atom bonds. By the development of these synthetic methods, substitution reactions to sp2 carbon and sp carbon are easily accomplished while it had been difficult to perform these transformations by classical synthetic reactions without using metal catalysts.
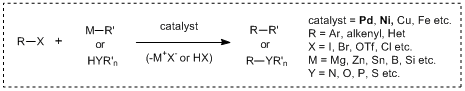
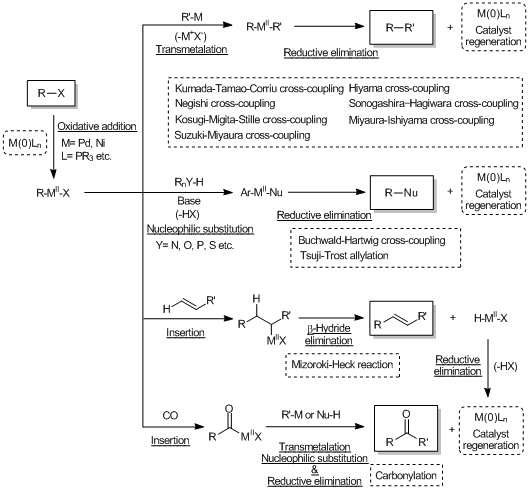
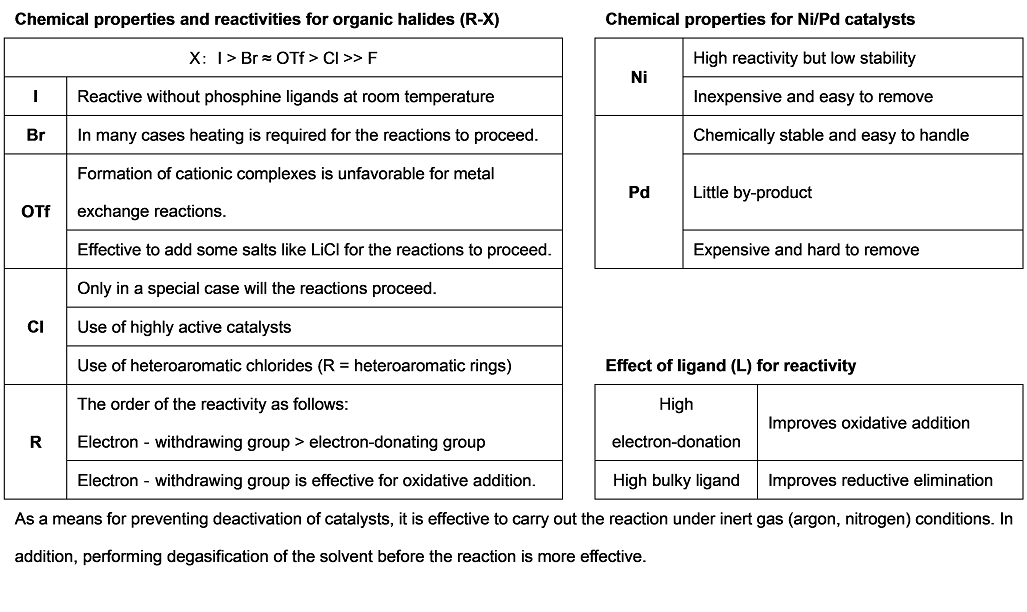
Recently, transition metal mediated cross-coupling reactions have been widely used as useful synthetic tools and applied to the synthesis of various functional molecules such as bioactive compounds and biaryls for liquid crystal materials. As a feature of these transformations, it is found that there are many name reactions for each kind of nucleophile used for coupling reactions. And then in 2010, for making a great contribution to develop the metal-based cross-coupling reactions, Richard F. Heck, Ei-ichi Negishi and Akira Suzuki jointly received the Nobel Prize in chemistry, verifying the usefulness of the transition metal mediated cross-coupling reactions. The following shows the synthetic properties of palladium/nickel catalyzed cross-coupling reactions commonly used with the chemical equations.
Cross-coupling reactions using palladium/Nickel catalysts
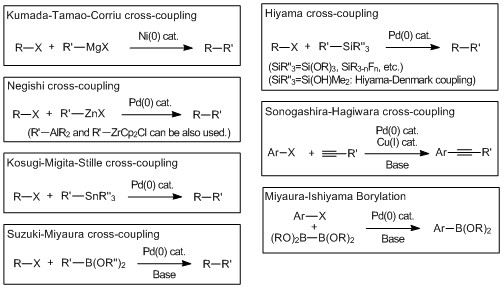
Cross-coupling reactions via the transmetalation
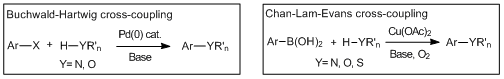
Cross-coupling reactions forming carbon-hetero atom bonds
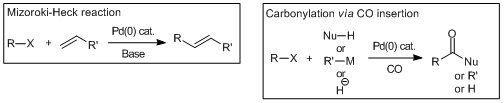
Cross-coupling reactions via the insertion
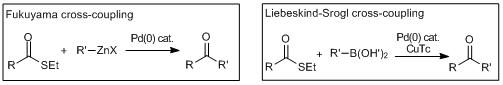
Cross-coupling reactions via the oxidative addition of thiolesters
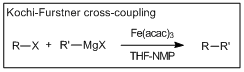
Cross-coupling reactions forming carbon sp3 – carbon sp3 bonds
Explore Cross-coupling Reaction using Transition Metal Catalysts [C-C Bond Formation] Categories
- Home
- Products
- Chemistry
- Catalysis and Inorganic Chemistry
- Cross-coupling Reaction using Transition Metal Catalysts [C-C Bond Formation]
- Home
- Products
- Chemistry
- Synthetic Reagents
- C-C Bond Formation [Synthetic Reagents]
- Cross-coupling Reaction using Transition Metal Catalysts [C-C Bond Formation]

