Maintenance Notice (4:00 AM November 1 - 9:30 AM November 1, 2025): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Published TCIMAIL newest issue No.199
Maintenance Notice (4:00 AM November 1 - 9:30 AM November 1, 2025): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Published TCIMAIL newest issue No.199
Maximum quantity allowed is 999
Cross-coupling reactions using late transition metal catalysts represented by nickel and palladium metals have been widely used for introducing various functional groups into unsaturated substances such as aromatic rings, alkenes, alkynes and so on. In these reactions, carbon-carbon bond forming reactions can be performed by the combination of electrophilic carbon species of aryl/vinyl halides and organometallic agents of Grignard reagents and organoboron compounds. Also, the use of nucleophilic hetero atoms such as phenols and amines is efficient to form carbon-hetero atom bonds. By the development of these synthetic methods, substitution reactions to sp2 carbon and sp carbon are easily accomplished while it had been difficult to perform these transformations by classical synthetic reactions without using metal catalysts.
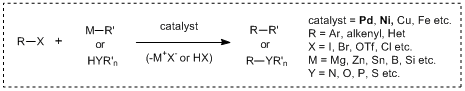
Recently, transition metal mediated cross-coupling reactions have been widely used as useful synthetic tools and applied to the synthesis of various functional molecules such as bioactive compounds and biaryls for liquid crystal materials. As a feature of these transformations, it is found that there are many name reactions for each kind of nucleophile used for coupling reactions. And then in 2010, for making a great contribution to develop the metal-based cross-coupling reactions, Richard F. Heck, Ei-ichi Negishi and Akira Suzuki jointly received the Nobel Prize in chemistry, verifying the usefulness of the transition metal mediated cross-coupling reactions. The following shows the synthetic properties of palladium/nickel catalyzed cross-coupling reactions commonly used with the chemical equations.
Cross-coupling reactions using palladium/Nickel catalysts
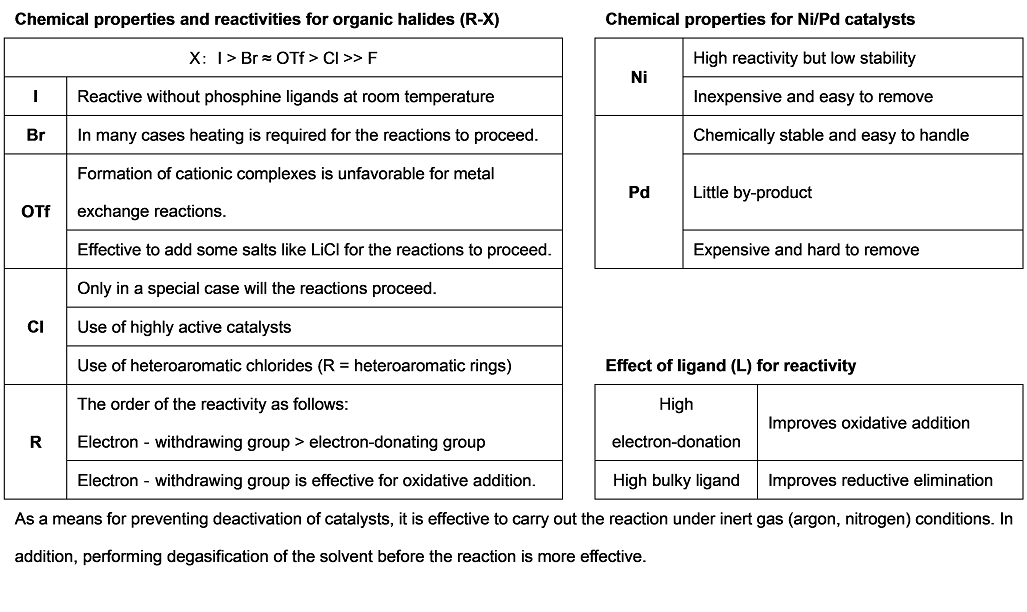
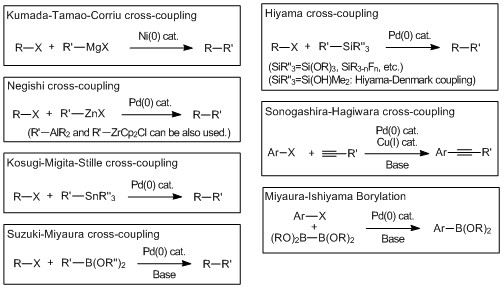
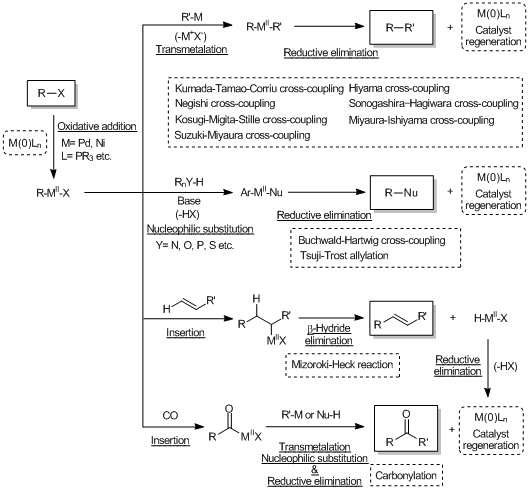
Cross-coupling reactions via the transmetalation
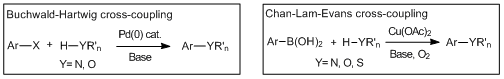
Cross-coupling reactions forming carbon-hetero atom bonds
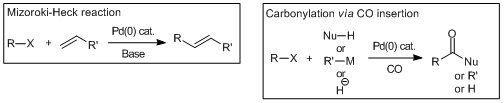
Cross-coupling reactions via the insertion
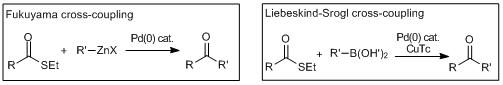
Cross-coupling reactions via the oxidative addition of thiolesters
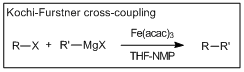
Cross-coupling reactions forming carbon sp3 – carbon sp3 bonds