Sign up for free shipping on all website orders, no minimum required, and get exclusive coupons!
Users can now ask questions to the AI chatbot by clicking the chatbot icon in the bottom-right corner of the website.
Maximum quantity allowed is 999
| Size | Unit Price | Philadelphia, PA | Portland, OR | Japan* | Quantity |
Shipping Information

|
|---|---|---|---|---|---|---|
| 25G |
$30.00
|
Contact Us | Contact Us | 30 |
|
|
| 500G |
$107.00
|
Contact Us | Contact Us | Ships within 1 month after ordering |
|
* Items in stock locally typically ship the same day of ordering. Items from Japan stock are able to ship from a US warehouse within 2 weeks after ordering. For additional estimated shipment times, please refer to the Shipping Simulation tool. Note: Excludes regulated items and items that ship on ice.
* To send your quote request for bulk quantities, please click on the "Request Quote" button. Please note that we cannot offer bulk quantities for some products.
*TCI frequently reviews storage conditions to optimize them. Please note that the latest information on the storage temperature for the products is described on our website.
| Product Number | I0604 |
Purity / Analysis Method 
|
>99.0%(T) |
| Molecular Formula / Molecular Weight | I__2 = 253.81 |
| Physical State (20 deg.C) | Solid |
Storage Temperature 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
| CAS RN | 7553-56-2 |
| Reaxys Registry Number | 3587194 |
| PubChem Substance ID | 87558517 |
| Merck Index (14) | 5014 |
| Appearance | Gray to Dark blue to Black powder to lump |
| Purity(Iodometric Titration) | min. 99.0 % |
| Melting Point | 114 °C |
| Boiling Point | 184 °C |
| Solubility in water | Insoluble |
| Degree of solubility in water | 0.3 g/l 20 °C |
| Solubility (soluble in) | Alcohol, Ether, Chloroform, Benzene |
| Pictogram |



|
| Signal Word | Danger |
| Hazard Statements | H312 + H332 : Harmful in contact with skin or if inhaled. H315 : Causes skin irritation. H319 : Causes serious eye irritation. H372 : Causes damage to organs through prolonged or repeated exposure. H317 : May cause an allergic skin reaction. H335 : May cause respiratory irritation. H410 : Very toxic to aquatic life with long lasting effects. |
| Precautionary Statements | P501 : Dispose of contents/ container to an approved waste disposal plant. P273 : Avoid release to the environment. P260 : Do not breathe dust. P270 : Do not eat, drink or smoke when using this product. P271 : Use only outdoors or in a well-ventilated area. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection. P272 : Contaminated work clothing must not be allowed out of the workplace. P391 : Collect spillage. P314 : Get medical advice/ attention if you feel unwell. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P333 + P313 : If skin irritation or rash occurs: Get medical advice/ attention. P362 : Take off contaminated clothing and wash before reuse. P302 + P352 + P312 : IF ON SKIN: Wash with plenty of water.Call a POISON CENTER/doctor if you feel unwell. P304 + P340 + P312 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Call a POISON CENTER/doctor if you feel unwell. P403 + P233 : Store in a well-ventilated place. Keep container tightly closed. P405 : Store locked up. |
| RTECS# | NN1575000 |
| UN Number (DOT-AIR) | UN3495 |
| Class (DOT-AIR) | 8/6.1 |
| Packing Group (TCI-A) | III |
| HS Number | 2801.20.0000 |
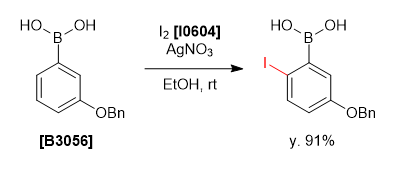
To a solution of 3-benzyloxyphenylboronic acid (342 mg, 1.50 mmol) and silver(I) nitrate (280 mg, 1.65 mmol, 1.1 eq.) in ethanol (4.5 mL) was added iodine (381 mg, 1.50 mmol, 1.0 eq.) in ethanol (7 mL) at rt and the mixture was stirred for 2 hours. The suspension was filtered and the filtrate was diluted with ethyl acetate (30 mL) and washed with water (30 mL) and brine (30 mL). The organic layer was dried over sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by column chromatography (ethyl acetate:hexane = 1:4 on silica gel) to give 2-iodo-5-benzyloxyphenylboronic acid as a white solid (483 mg, 91%).
Silver salts precipitated as the reaction proceeded.
The reaction mixture was monitored by UPLC.
2-Iodo-5-benzyloxyphenylboronic Acid
1H NMR (270 MHz, CDCl3); δ 8.22 (s, 2H), 7.60 (d, 1H, J = 8.6 Hz), 7.47-7.31 (m, 5H), 6.90 (d, 1H, J = 3.5 Hz), 6.76 (dd, 1H, J = 8.6, 3.0 Hz), 5.08 (s, 2H).

To a mixture of iodine (0.416 g, 1.63 mmol), PPh3 (0.416 g, 1.64 mmol) and Imidazole (0.223 g, 3.27 mmol) in CH2Cl2 (3.3 mL) at 0 ºC was added a solution of 2-phenylethyl alcohol (0.196 mL, 1.64 mmol) in CH2Cl2 (3.3 mL). The reaction mixture was stirred at 0 ºC for 1 h. The reaction mixture was added aqueous solution (10 mL) of sodium thiosulfate pentahydrate (2.0 g, 8.19 mmol) and then extracted by CH2Cl2. The organic layer was dried over Na2SO4 and concentrated under reduced pressure, and purified by silica gel column chromatography (hexane:ethyl acetate = 9:1) to give (2-iodoethyl)benzene (0.360 g, 95% yield) as a colorless liquid.
The reaction mixture was monitored by TLC (hexane:ethyl acetate= 9:1, Rf = 0.6).
1H NMR (400 MHz, CDCl3); δ 7.33-7.17 (m, 5H), 3.34 (t, 2H, J = 7.6 Hz), 3.17 (t, 2H, J = 7.6 Hz).
13C NMR (101 MHz, CDCl3); δ 140.6, 128.6, 128.3, 126.8, 40.3, 5.62.


The requested SDS is not available.
Please Contact Us for more information.
A sample C of A for this product is not available at this time.

The requested analytical chart is not available. Sorry for the inconvenience.